╠ř

ACTEAST is necessary to improve outcomes for patients, learn more about ACTEAST. ╠řWe have leading researchers, clinicians and health care administrators from across Atlantic Canada on our team, view our full ACTEAST Team.╠ř
╠ř
What is ACTEAST?
ACTEAST aims to improve access and efficiency of treatment for ischemic stroke across all 4 provinces in Atlantic Canada.
Stroke is a devastating disease, as it is the leading cause of severe physical disability. Ischemic stroke is the most common form of stroke, and it is treatable with medical treatment (thrombolysis)╠ř and a new minimally invasive surgical procedure (Endovascular Thrombectomy - EVT). These treatments can transform lives, but minutes matter for improving outcomes.
ACTEAST aims to increase the proportion of ischemic stroke patients receiving treatment, and improve the efficiency of treatment.╠ř We will carry out this work across all of the Atlantic provinces: Nova Scotia, New Brunswick, Prince Edward Island, and Newfoundland & Labrador.
Why is this Important?
In a typical acute ischemic stroke╠ř1.9 million neurons die every minute.╠řTime is Brain.
In ischemic stroke patients, opening the blocked artery as soon as possible saves brain. Treating patients faster with both alteplase (tPA) and endovascular thrombectomy (EVT) improves the chance that a patient can return home with no or little disability. ACTEAST was a 3-year project.╠ř
What are the Benefits?
The potential benefit for patients experiencing ischemic stroke is profound. It is anticipated that 10-20% of ischemic stroke patients will have improved outcomes, which means that up to 550 more patients each year in Atlantic Canada can return to their homes with no or little disability, and utilize much less rehabilitation and long-term care services.
What were the goals?
- Increase the proportion of ischemic stroke patients that receive alteplase or EVT by 5%
- Reduce the time to treatment for both alteplase and EVT
- Reduce the door-to-needle time for alteplase treatment to a median of 30 minutes
- Reduce the door-to-groin-puncture time for EVT treatment to a median of 60 minutes
- Reduce the door-in-door-out time for patients transferred for EVT to a median of 50 minutes
- Reduce the time from first medical treatment to needle and groin puncture
How Was Improvement Made?
An╠řImprovement Collaborative╠řwas be carried out three times across Atlantic Canada. The Improvement Collaborative uses alternating face-to-face workshops (called Learning Sessions) and action periods to test and implement changes at local hospitals.
Each Improvement Collaborative was 6-months long, and they were carried out at different times across the region.
The order of participation will be as follows:
- Stroke centres across Nova Scotia
- Stroke centres across New Brunswick and Prince Edward Island
- Stroke centres across Newfoundland and Labrador
All Primary and Comprehensive stroke centres will participate in an╠řImprovement Collaborative.╠ř╠řThe Collaborative had two╠řLearning Sessions. ╠řThe╠řLearning Sessions╠řinvolve sharing information with hospital teams and facilitation of cross-site learning.╠řAfter each Learning Session, sites tested and implemented improvement during the╠řAction Period. Alongside world-leading stroke neurologists, tthe research team supported with╠řsite visits╠řand bi-monthly webinars to assist with╠ř improvement efforts during the Action Period.╠ř
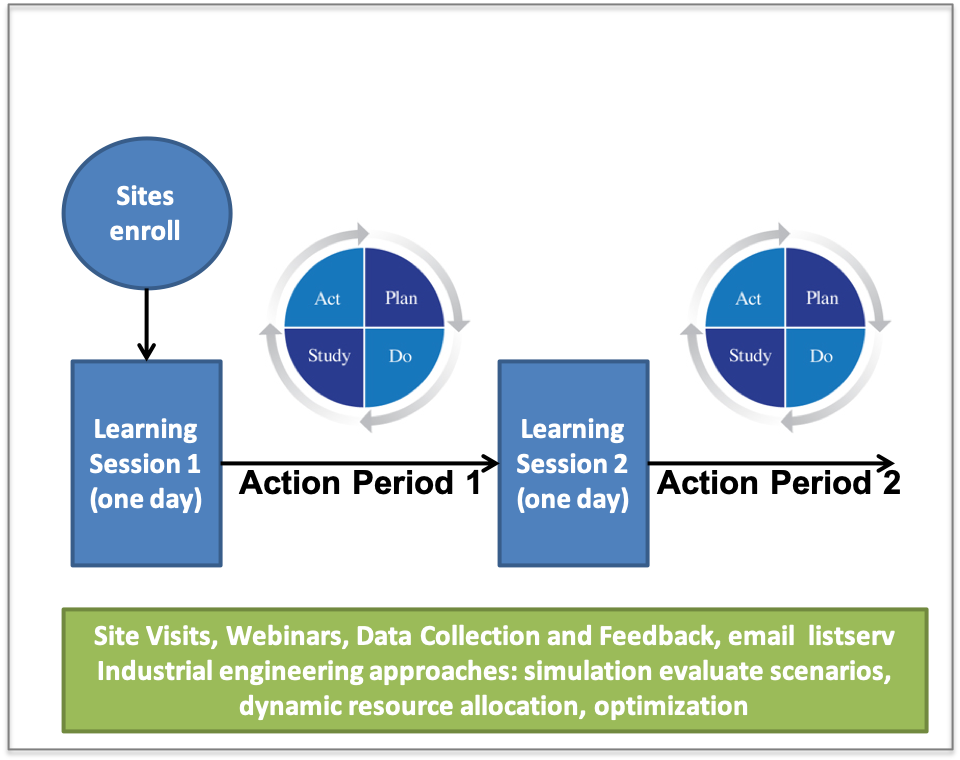
The Improvement Collaborative Process:
- The Improvement Collaborative starts with site enrolling,╠ř
- Teams then participate in the first Learning Session
- The first Action Period follows where changes are trialed using PDSA (Plan Do Study Act) cycles
- The second Learning Session follows the first Action Period
- The final step is the second Action Period
Here is a diagram that shows Improvement Collaborative process:
How Was Trial Conducted?
ACTEAST was carried out as a Stepped Wedge Trial. ╠řAs shown in the Figure below, the Improvement Collaborative (Intervention) was rolled out in phases/waves, thus the clusters are for╠řwhen╠řsites receive the intervention, not╠řif╠řthey receive the intervention. Each cluster is given the intervention at different times, and sites are assigned to the cluster based on their province.
Sites |
Lead in Phase
|
Phase 1
|
Phase 2
|
Phase 3
|
Phase 4
|
|---|---|---|---|---|---|
| Cluster 1 [NS; n=10] |
Retrospective Data Collection |
Intervention | Yes | Yes | Yes |
| Cluster 2 [NB,PEI; n=13] |
Retrospective Data Collection |
No | Intervention | Yes | Yes |
| Cluster 3 [NL; n=10] |
Retrospective Data Collection |
No | No | Intervention | Yes |
The yellow periods are patients treated without the intervention (pre-intervention). The green periods are patients treated with the intervention (post-intervention). In the evaluation, the orange and green periods are compared for each of our key measures
╠ř
What are the Benefits?
The potential benefit for patients experiencing ischemic stroke is profound. It is anticipated that 10-20% of ischemic stroke patients will have improved outcomes, which means that up to 550 more patients each year in Atlantic Canada can return to their homes with no or little disability, and utilize much less rehabilitation and long-term care services.

