GCāMS studies
Understanding electrolyte additive function using GC-MS
In order to understand how electrolyte additives work, the chemical reactions that occur at the electrode surfaces must be determined. When additives react at each electrode, they create insoluble reaction products at the electrode surfaces and soluble products in the electrolyte.
Gas Chromatography/Mass Spectrometry is a very powerful technique to identify the organic compounds present in any sample.Ā By analyzing the electrolyte of Li-ion cells during charge-discharge cycling, the fate of electrolyte components can be studied.Ā
The Use of Gas Chromatography-Mass Spectrometry (GC-MS) to Study the Changes in the Electrolyte of Li-ion Cells during Operation
The composition of the electrolyte in Li-ion cells changes when cells undergo their very first charge1 and also when cells are subjected to different operations (e.g. charge-discharge cycling, storage)2. GC-MS is a powerful tool to determine the components in the electrolyte of Li-ion cells semi-quantitaively and qualitatively.Ā Figure 2a shows an example of the use of GC-MS to study how electrolyte additives, called enablers, such as vinylene carbonate (VC), fluoroethylene carbonate (FEC) and others are consumed during the very first charge of Li-ion cells. In addition, Figure 2b shows that one of the majority components of the electrolyte, ethyl methyl carbonate, undergoes transesterification to dimethyl carbonate and diethyl carbonate if the enable content is too low. Amazingly, the Li-ion cell is a little chemical factory! Ā More details can be obtained by reading reference 3.

Figure 1 - The GC-MS in the Dahn lab is a Bruker 436-GC coupled to a Bruker Scion single-quadrupole mass spectrometer
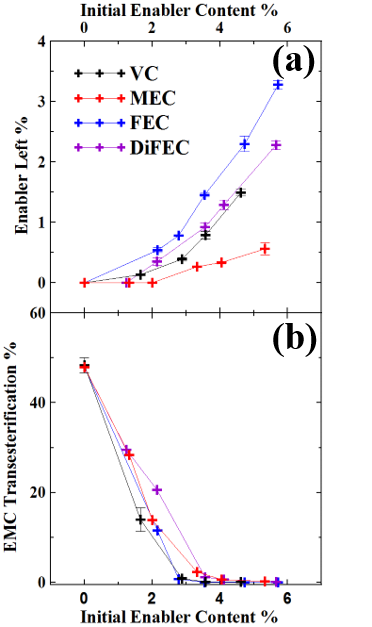
Figure 2Ā
References:
- K. Xu, Chem. Rev., 114, 11503ā11618 (2014).
- S. E. Sloop, J. B. Kerr, and K. Kinoshita, J. Power Sources, 119ā121, 330ā337 (2003)
- L. Ma, S.L. Glazier et al., J. Electrochem. Soc. 164,A5008-A5018 (2017)
